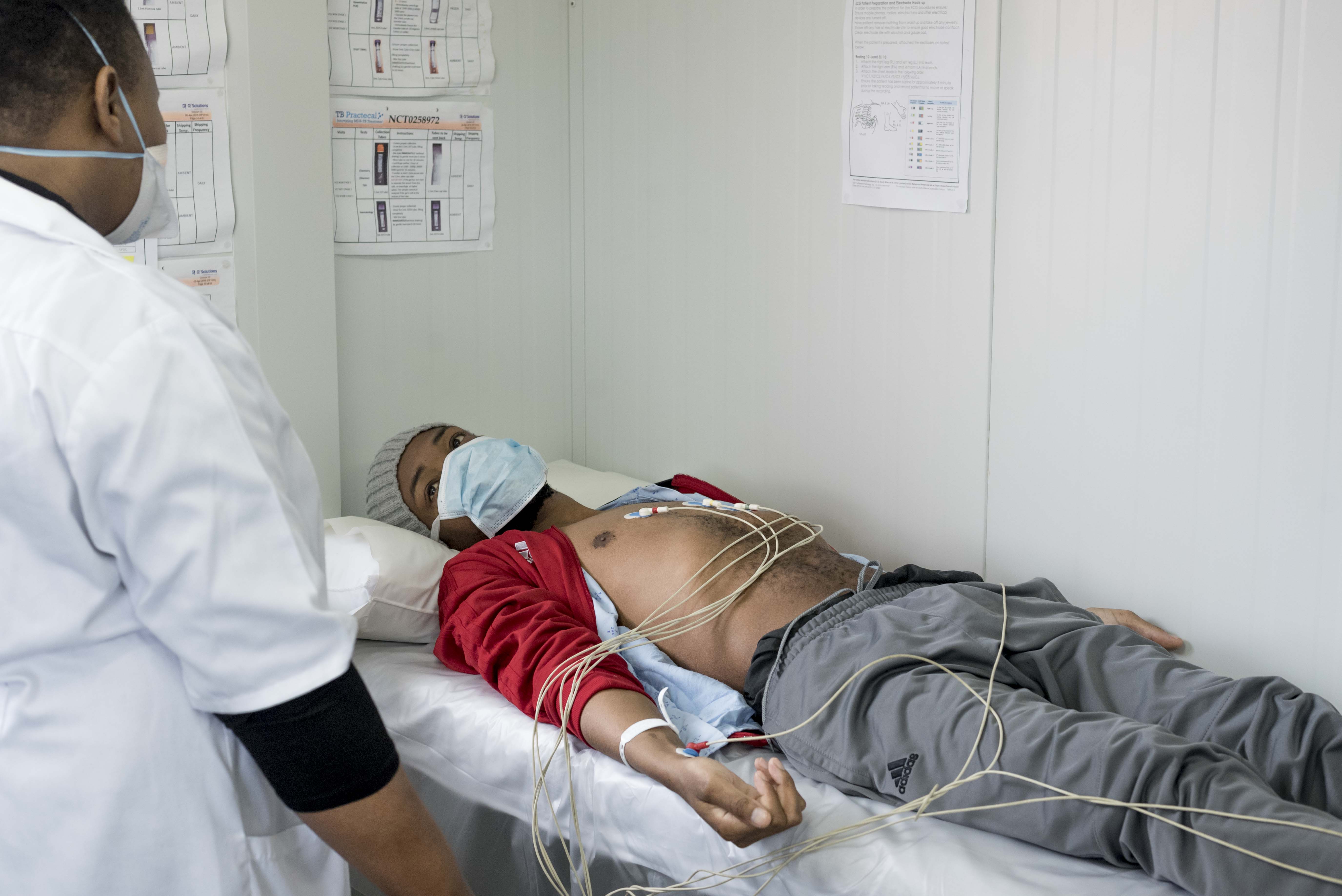
TB PRACTECAL: “Having a shorter treatment would mean so much for people like me”
Bonginkosi Emmanuel Mkhize is the 240th patient to enrol onto TB PRACTECAL – an MSF-supported clinical research project to find short, tolerable and effective treatments for people with multidrug-resistant tuberculosis (MDR-TB).
MDR-TB is a strain of the tuberculosis bacterial infection that does not respond to first-line antibiotics and can require an arduous two-year treatment.
“When I found out that I had the same TB as my sister, I wasn’t surprised because I was so sick – just like her”
“I decided to take part in TB PRACTECAL because I wanted to make a difference for my children’s future,” says Bonginkosi.
His enrolment marks an important milestone for the project as it signifies the end of the initial safety and efficacy stage of the trial and the beginning of the second and final stage.
Big sister
Before he fell sick, 32-year-old Bonginkosi worked as an electrician repairing fridges to provide for his young family in the city of Durban, South Africa.
Six years ago, his big sister contracted MDR-TB.
“They didn’t let us see her, and she died,” says Bonginkosi.
“She was just 30-years-old. Her death really affected me, it set me back. I couldn’t see her before she died. Growing up it was always just her and me.”
“When I found out that I had the same TB as my sister, I wasn’t surprised because I was so sick – just like her.”
According to the World Health Organization, an estimated 558,000 people developed MDR-TB in 2017 alone. Each year around 20,000 drug-susceptible-TB & MDR-TB patients are treated in MSF-supported projects in over 25 different countries around the world.
Family support
Bonginkosi’s family have been supportive of his decision to take part in the trial.
“When I told my wife that I had to stay in hospital for a long time, she couldn’t stop crying. But she understands.
“My whole family visit and support me. I tell everyone that they must go get checked up. I made my whole family get checked up.”
Like many patients with MDR-TB, the effects of the disease have taken a huge toll on Bonginkosi’s life.
“I love my wife and my children. But now I can’t be with them. And I can’t work so I have no income. This is how TB has affected my daily life. So, it’s hard.”
Reducing side effects
The TB PRACTECAL trial objectives focus specifically on the huge physical, psychological and socio-economic effects that current MDR-TB treatments have on patients.

“This complex global trial is only able to function due to the huge amount of work and dedication shown by over 60 research staff working in seven hospitals across three countries"
By testing six-months, all-oral, once-a-day regimens, TB PRACTECAL hopes to radically improve the lives of people suffering from the disease.
The current MDR-TB treatment involves taking up to 20 pills daily, for between nine and 24 months.
"It’s an opportunity to treat patients holistically, and I’ve been able to form closer, more meaningful relationships with them,” says Dr Cindy Narasimooloo, who recruited Bonginkosi onto the trial.
She works for THINK, a research organisation which MSF has partnered with to run the trial in Durban, South Africa.
“It’s exciting to be part of something ground-breaking,” she says.
The magic number
Bonginkosi’s enrolment in the programme marks the transition point between the two main stages of the trial.
During stage one, three combinations of new TB drugs were tested to see which combinations had the best results for patients when taken over a two-month period.
In stage two, these combinations when taken for the full six months will be directly compared to the current standard treatment for MDR-TB.
“This analysis of stage one data will – for the first time – give us rigorous evidence on the effectiveness of these new drugs. We will be one step closer to revolutionising treatment for MDR-TB the world over,” says Emil Kazounis, TB PRACTECAL clinical trial manager.
“The reason why ‘240’ is the magic number is that it means we now have enough data to make accurate decisions regarding which combination will be taken forward to be tested in stage two.”
Towards 2022
“This complex global trial is only able to function due to the huge amount of work and dedication shown by over 60 research staff working in seven hospitals across three countries,” says Emil.
“There is, of course, a lot of work still to do - we’ll be recruiting 630 patients in total to the trial, and we’re expecting the final results in early 2022. But this is a very exciting time for the MSF project team and for all the staff working on the trial.”
Although he has only just started a new treatment regimen, Bonginkosi also remains hopeful:
“Having a shorter treatment for MDR TB would mean so much for me and people like me.”
MSF and tuberculosis
Every year, over 10 million people develop active TB and 1.6 million die from it.
TB is often thought of as a disease of the past but a recent resurgence and the spread of drug-resistant forms make it very much an issue of the present day and age.
Almost half a million people develop multidrug-resistant strains of the disease every year. Today, TB is one of the three main killer infectious diseases, along with malaria and HIV/AIDS.